20210323 DNA Inside an Adenovirus The researchers added the gene for the coronavirus spike protein to another virus called Adenovirus 26. A dose-escalation open-label non-randomised first-in-human trial.
 Retroviral And Adenoviral Vectors Retroviruses Based On Moloney Murine Download Scientific Diagram
Retroviral And Adenoviral Vectors Retroviruses Based On Moloney Murine Download Scientific Diagram
20200917 AdHu5 is the most commonly used human adenovirus serotype for the development of the tuberculosis vaccine.

What is adenovirus vector technology. Safety tolerability and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine. Adenoviral vectors are non-enveloped double-stranded DNA vectors. To make an adenoviral vector scientists take an adenovirus and remove any genetic material.
The viral vector acts as a delivery system providing a means to invade the cell and insert the code for a different virus antigens the pathogen youre trying to vaccinate against. COVID-19 viral vector vaccines inject a harmless adenovirus vector which carries unique genetic information from the COVID-19 virus to human cells. They contain a double-stranded DNA genome.
Johnson use adenovirus vector platform vaccine Xu Shuchang does not rule out thrombosis and advocates not to buy it temporarily AstraZeneca It was stated yesterday that the plan will remain unchanged and will continue to work closely with the Hong Kong and Macau governments to provide AstraZeneca vaccines to Hong Kong and Macau. In order to make this 05 ml of transparent liquid effective to prevent the new crown virus from infecting the human body and defeat the new crown epidemic that is still spreading around the world vaccine research and development workers have devoted. 20210323 The Oxford-AstraZeneca vaccine for Covid-19 is more rugged than the mRNA vaccines from Pfizer and Moderna.
20201218 Adenovirus -vectored vaccines are the other technology that has been close behind the mRNA-based vaccines in clinical testing. Of the six vaccines backed by Operation Warp Speed the adenovirus. First the vector not the virus that causes COVID-19 but a different harmless virus will enter a cell in our body and then use the cells machinery to produce a harmless piece of the virus that causes COVID-19.
In the case of Johnson. 20210410 AstraZeneca and Johnson. The virus itself is harmless and by getting the cells only to produce antigens the body can mount an immune response safely without developing disease.
DNA is not as fragile as RNA and the adenoviruss tough protein coat helps protect the. Many studies conducted in the early 1990s characterized the neural tropism of adenoviral vectors in vitro and in vivo. Johnson is seeking emergency use authorization for what would become the USs first one-dose and non-mRNA COVID vaccine.
To accommodate larger recombinant genes up to 8 Kb 1st generation adenoviruses are both E1 and E3 deleted E1E3 since the E3 region is not essential for in vitro viral growth. AAV is of particular interest to gene therapists due to its apparent limited capacity to induce immune responses in humans a factor which should positively influence vector transduction efficiency while reducing the risk of any. 20210413 Adenovirus-based vaccines use the adenovirus as a vector to deliver an antigen.
The dose of a vaccine is usually 05 ml. These viruses have a wide range of vertebrate hosts including some species of birds frogs sturgeon cattle and mammals including humans. 20210304 Adenoviruses are a group of viruses in the taxonomic family Adenoviridae.
20210414 A vector is a virus shell that researchers can use to package up and deliver a target from another virus. The most widely studied adenovirus-based tuberculosis vaccine is E1E2 depleted AdHu5. There are more than 50 distinct adenovirus serotypes known to infect humans.
Recombinant Adenovirus DE1E3 To ensure replication deficiency of the virus the E1 region is deleted allowing it to safely be used as a gene delivery tool. It employs adenovirus vectors a technology that has been used. 20210413 Viral vector vaccines use a modified version of a different virus the vector to deliver important instructions to our cells.
Johnsons vaccine part of the adenoviruss genetic. 20210302 The virus vector being used in the Johnson. Johnson and AstraZeneca vaccines is an adenovirus a common type of virus that typically causes mild cold symptoms when it infects someone.
20201019 Use of adenovirus type-5 vectored vaccines. 20201231 How Adenovirus Vector COVID-19 Vaccine Ad5-nCoV works. And subsequent advanced trials.
Delivering The Goods The Scientist Magazine
 The Adenovirus System Introduction Abm Inc
The Adenovirus System Introduction Abm Inc
 Recent Advances In Genetic Modification Of Adenovirus Vectors For Cancer Treatment Yamamoto 2017 Cancer Science Wiley Online Library
Recent Advances In Genetic Modification Of Adenovirus Vectors For Cancer Treatment Yamamoto 2017 Cancer Science Wiley Online Library
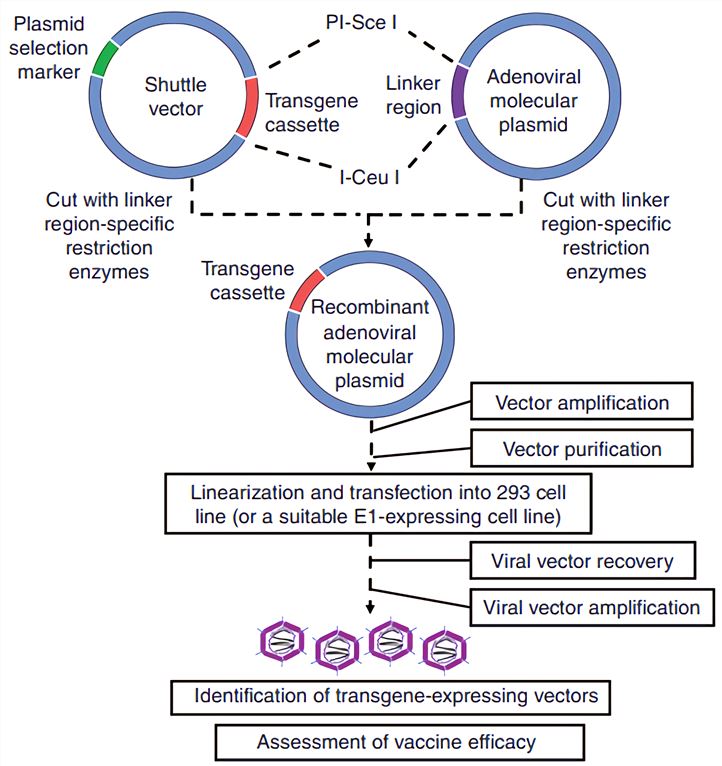 Adenovirus Ad As Vaccine Vectors Creative Biolabs
Adenovirus Ad As Vaccine Vectors Creative Biolabs
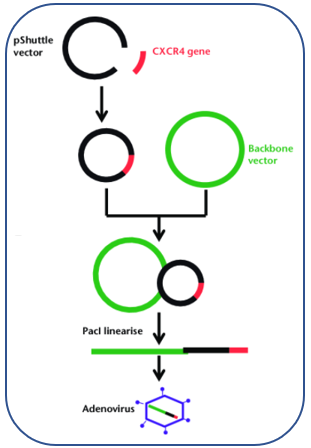 Adenovirus Vector Creative Biolabs
Adenovirus Vector Creative Biolabs
 A Simplified System For Generating Recombinant Adenoviruses Pnas
A Simplified System For Generating Recombinant Adenoviruses Pnas
 Immunology Of Adenoviral Vectors In Cancer Therapy Molecular Therapy Methods Clinical Development
Immunology Of Adenoviral Vectors In Cancer Therapy Molecular Therapy Methods Clinical Development
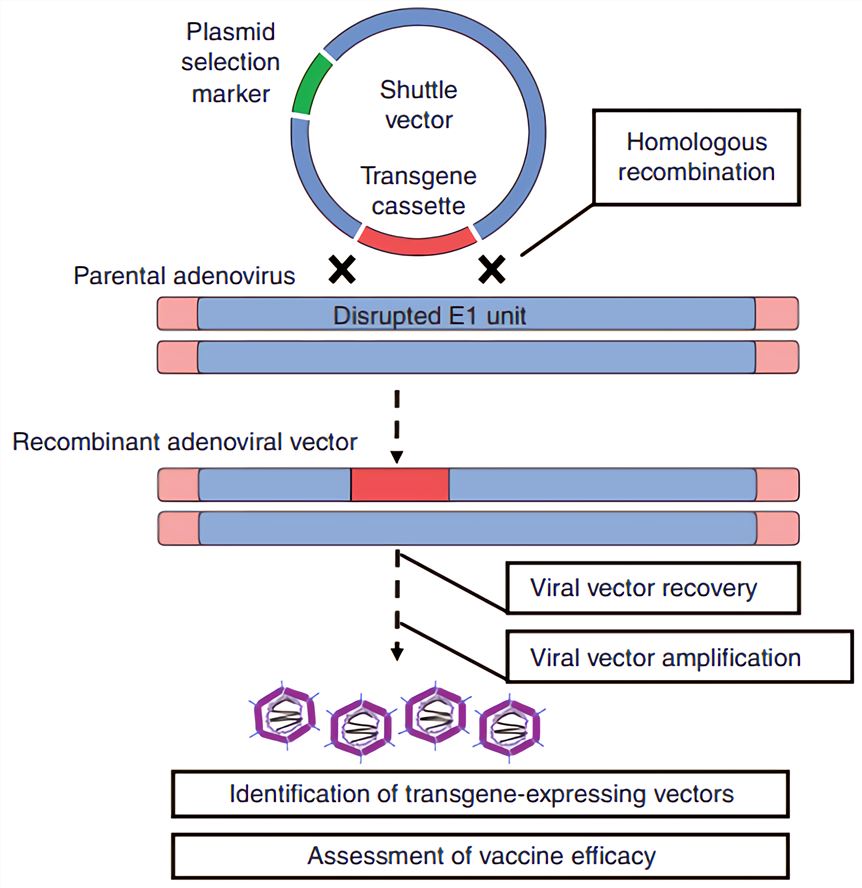 Adenovirus Ad As Vaccine Vectors Creative Biolabs
Adenovirus Ad As Vaccine Vectors Creative Biolabs
 Structure Of Adenoviral Vectors And Principle Of Adenovirus Production Download Scientific Diagram
Structure Of Adenoviral Vectors And Principle Of Adenovirus Production Download Scientific Diagram
 Ad Ding Value The Science Behind Adenovirus Vector Vaccines Research America
Ad Ding Value The Science Behind Adenovirus Vector Vaccines Research America
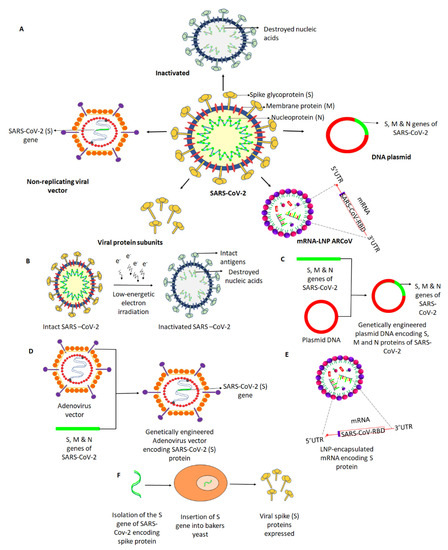 Vaccines Free Full Text Platforms Exploited For Sars Cov 2 Vaccine Development Html
Vaccines Free Full Text Platforms Exploited For Sars Cov 2 Vaccine Development Html
 Adenovirus Vector Based Vaccine Strategy For Influenza Download Scientific Diagram
Adenovirus Vector Based Vaccine Strategy For Influenza Download Scientific Diagram
How Pfizer S Mrna Coronavirus Vaccine Compares To Other Us Candidates
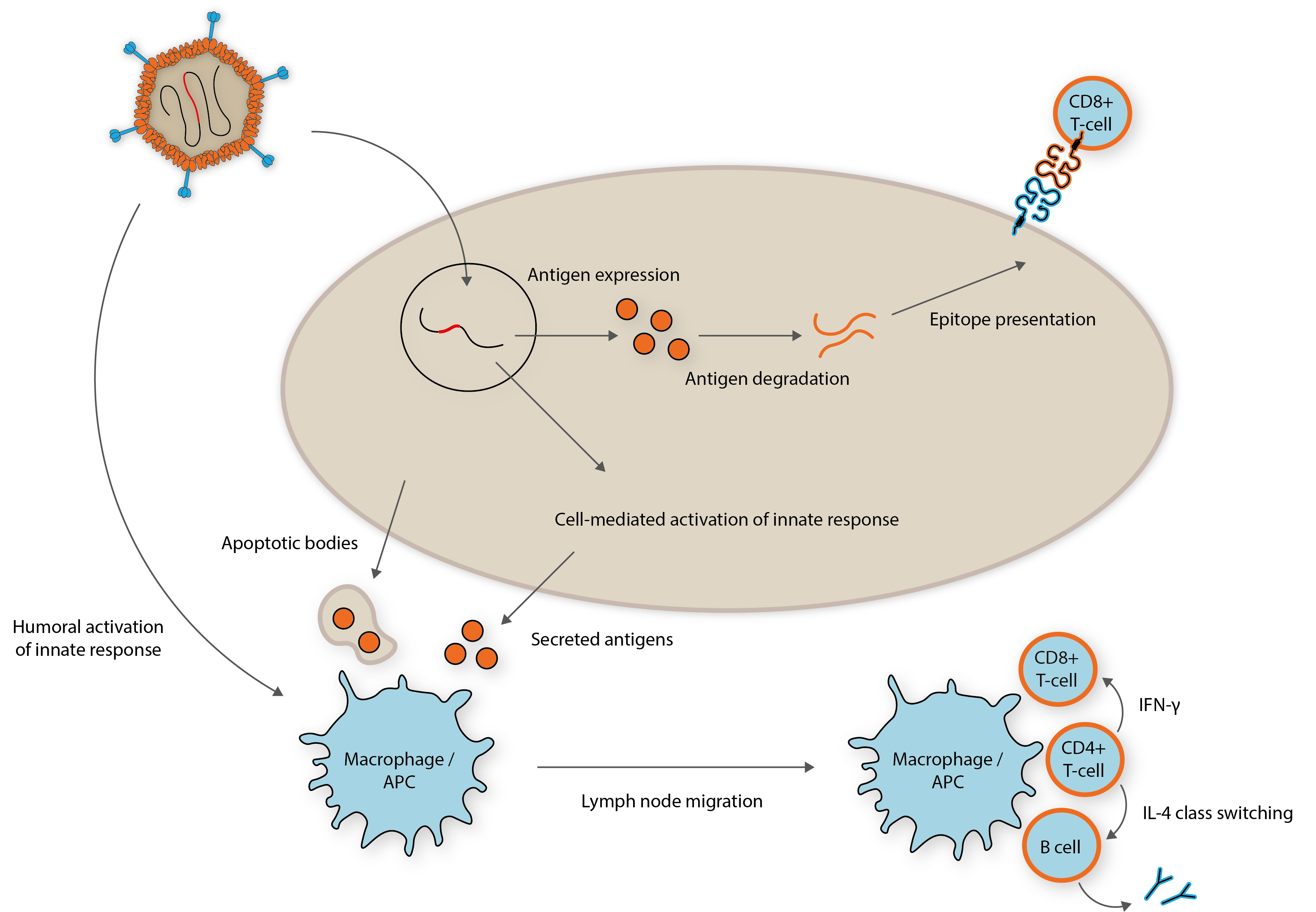 Repurposing Adenoviruses As Vectors For Vaccines The Native Antigen Company
Repurposing Adenoviruses As Vectors For Vaccines The Native Antigen Company
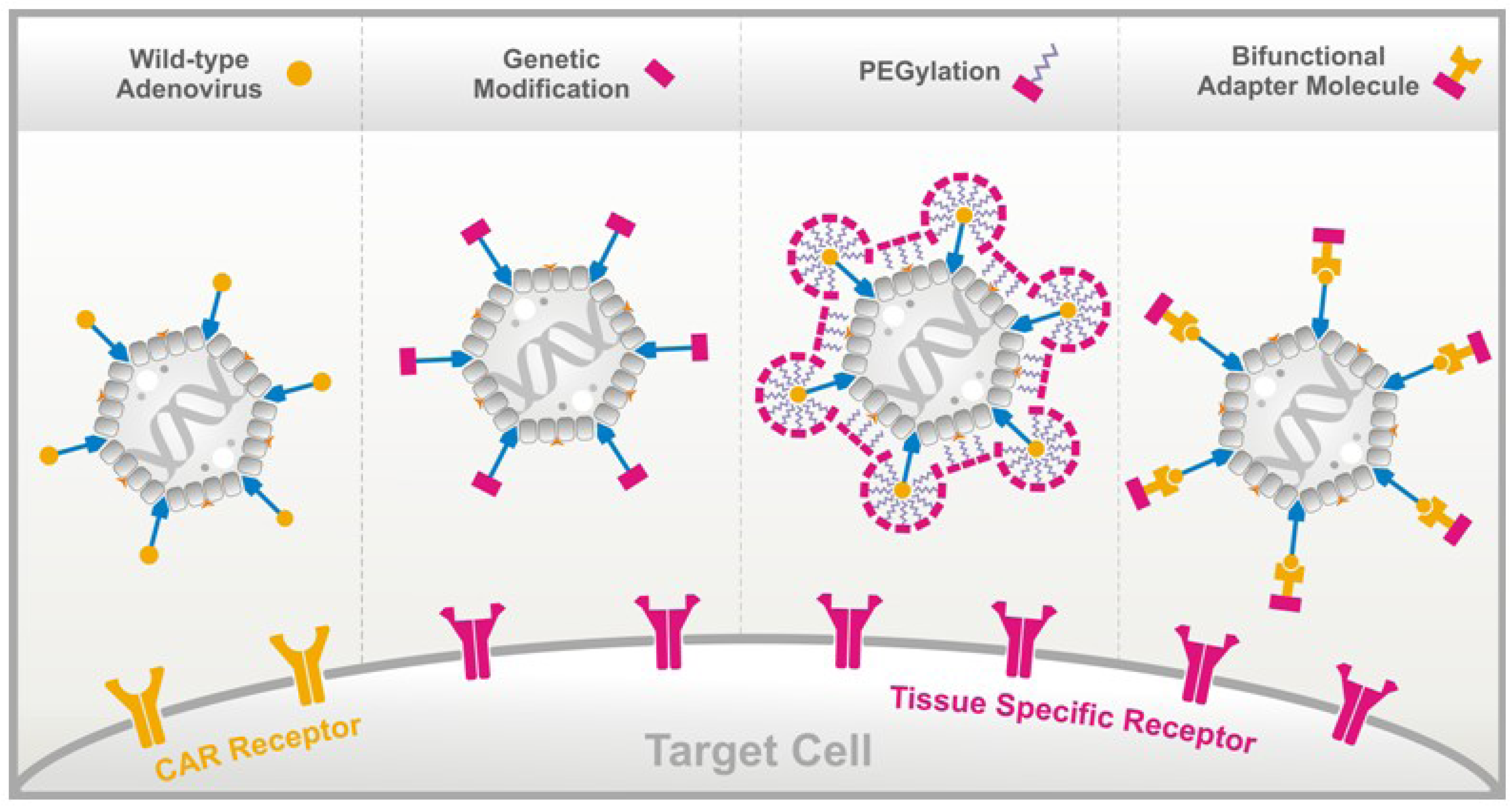 Viruses Free Full Text Peptide Based Technologies To Alter Adenoviral Vector Tropism Ways And Means For Systemic Treatment Of Cancer Html
Viruses Free Full Text Peptide Based Technologies To Alter Adenoviral Vector Tropism Ways And Means For Systemic Treatment Of Cancer Html
 Adenovirus The First Effective In Vivo Gene Delivery Vector Human Gene Therapy
Adenovirus The First Effective In Vivo Gene Delivery Vector Human Gene Therapy
 Adenovirus Vector System Adenovirus Production And Transduction Genemedi
Adenovirus Vector System Adenovirus Production And Transduction Genemedi
 Development Of A Freeze Dried Ebola Expressing Adenoviral Vector Unexpected Findings And Problems Solved Bioprocess Internationalbioprocess International
Development Of A Freeze Dried Ebola Expressing Adenoviral Vector Unexpected Findings And Problems Solved Bioprocess Internationalbioprocess International
 Methods And Clinical Development Of Adenovirus Vectored Vaccines Against Mucosal Pathogens Molecular Therapy Methods Clinical Development
Methods And Clinical Development Of Adenovirus Vectored Vaccines Against Mucosal Pathogens Molecular Therapy Methods Clinical Development
